NCPA PAC Raised $35,000 at NCPA Annual Convention: The National Community Pharmacists Association Political Action Committee (NCPA PAC) held a fundraising reception on Sunday, October 11, 2015 during the NCPA Annual Convention and raised $35,000 for the NCPA PAC. Thank you to all NCPA PAC contributors and a special thank you to our 2015 PAC MVP’s who donate the legal maximum of $5,000 annually to the NCPA PAC:
- Brad Arthur, Buffalo, NY
- Shelley Bailey, Portland, OR
- Jeff Carson, San Antonio, TX
- John Carson, San Antonio, TX
- Brian Caswell, Baxter Springs, KS
- Hugh Chancy, Hahira, GA
- Danny Cottrell, Brewton, AL
- Stephen Giroux, Middleport, NY
- Robert Greenwood, Waterloo, IA
- H. Edward Heckman, Stoughton, WI
- B. Douglas Hoey, Alexandria, VA
- Lowell Irby, Artesia, NM
- Edmund Horton, Stephenville, TX
- Tony Ogden, Pasadena, TX
- Bill Osborn, Miami, OK
- Michael Schultz, Earth City, MO
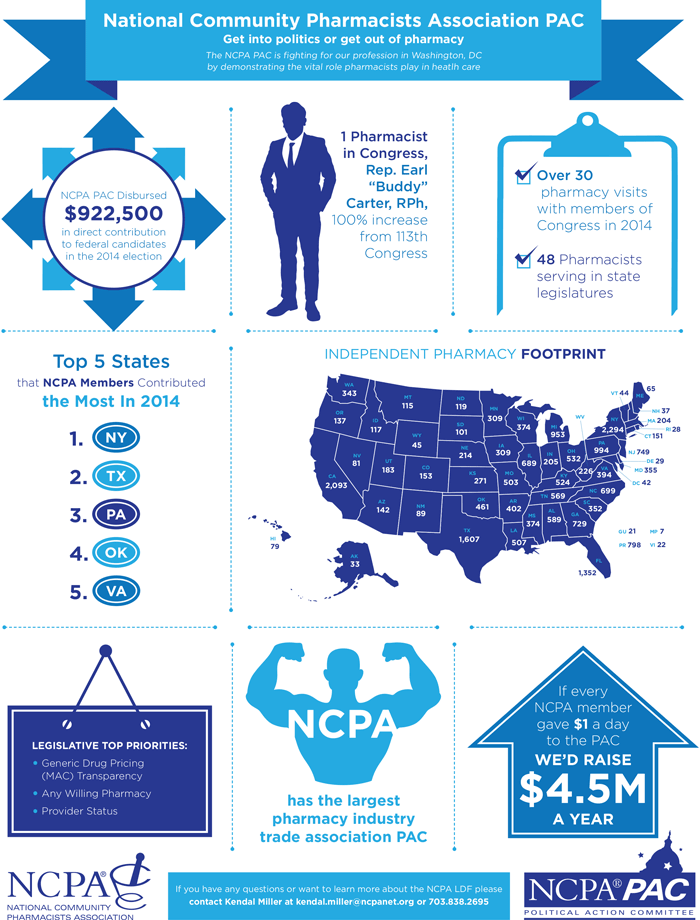
Also, please see the new NCPA PAC infographic below and help spread the word among your colleagues on the importance of contributing to the NCPA PAC. The infographic is also available as a printable PDF in the "Documents to Download" section below the article. The NCPA PAC is your voice on Capitol Hill, the support of NCPA members to the NCPA PAC enables our government affairs team to meet with and educate members of Congress to raise the profile of community pharmacy’s increasing value to health care.
Members of Congress Urge CMS to Finalize DIR Guidance: Last year CMS proposed guidance that would help to ensure that Part D plan sponsors consistently report pharmacy price concessions. Current variations in the treatment of costs and price concessions affect beneficiary cost sharing, CMS payment to plans, federal reinsurance and low income cost sharing (LIS), manufacturer coverage gap discount payments, and plan bids. Some Part D plans sponsors have manipulated how and when to report certain price concessions received from pharmacies, and as a result beneficiaries could be relying on inaccurate data when using Medicare Plan Finder. In addition, the ability of CMS to oversee PBMs to protect taxpayer funds from misuse is greatly undermined. NCPA thanks the eleven members of Congress who recently wrote to CMS, supporting the CMS proposed guidance and asking it to be finalized in the near future. By requiring uniform reporting standards, pharmacy price concessions can be consistently approximated at point of sale and appropriately reported by Part D plans.
NCPA on Record with Department of Defense Regarding New Mail Order Requirements: NCPA recently submitted formal comments to DoD related to the interim final rule implementing Section 702 of the Carl Levin and Howard P. “Buck” McKeon National Defense Authorization Act for Fiscal Year 2015. Section 702 states that beginning October 1, 2015, the pharmacy benefits program shall require eligible covered beneficiaries generally to refill non-generic prescription maintenance medications through military treatment facility pharmacies or the national mail-order pharmacy program. DoD is currently considering possible revisions to this rule for the second year of the program, and NCPA recommendations included the following:
- TRICARE should make clear in amended Section 199.21 that covered maintenance medications include non-generic medications only
- TRICARE should require patient consent confirming need for medication is documented prior to each delivery via the TRICARE mail order program
- DoD conduct and publicly release patient satisfaction surveys in specific regards to the program. This will allow an honest assessment of patient experience with the program and will help to identify any problems related to a lack of coordinated medication therapy care. This recommendation goes hand in hand with a recent Government Accountability Office (GAO) study examining the TRICARE for Life pilot.
- All communications from the TRICARE pharmacy benefits manager to beneficiaries should include information on how to obtain a waiver
Medicare 2016 Part D Open Enrollment Underway… NCPA Resources Can Help: As Medicare beneficiaries begin reviewing their Part D plan choices for next year, pharmacists are sure to get an a lot of questions. To help you in assisting patients, NCPA has created a number of resources, including two new ones:
- Pharmacist Quick Reference Guide
- 2016 Medicare Part D Update and FAQs
- Top 10 Part D Plans—Preferred Network Status of Independents
- 2016 Medicare Part D Evaluation Tool
- Summary of Changes for Calendar Year 2016—Provisions of Interest to Community Pharmacy
- FAQ on Direct and Indirect Remuneration (DIR) Fees [New]
- Bag stuffer for Patients and Caregivers about Annual Enrollment Period [New]
The 2016 Medicare Part D Evaluation Tool has a new feature this year as we have added a designator to indicate if a DIR is charged to network pharmacies.
NCPA Meets with White House on Medicaid Generic Rx Reimbursements: NCPA told White House budget and regulatory affairs officials as well as health policy representatives working on Medicaid generic drug reimbursement changes that the Average Manufacturer Price-based Federal Upper Limits under consideration "in general are fundamentally flawed. They are not an accurate reimbursement metric that should be used to set state Medicaid pharmacy reimbursement on a drug-by-drug basis." At the private meeting, NCPA expressed cautious optimism about using the National Average Drug Acquisition Cost price bench mark instead.
Federal Judge Narrows 340B Discount Drug Program: A federal court last week struck down guidance issued by the Health Resources and Services Administration (HRSA) that required drug manufacturers to provide discounts between 20% and 50% on orphan drugs used for non-orphan conditions to 340B facilities serving vulnerable patients. The rule allowed 340B-eligible rural and cancer hospitals to purchase high-cost orphan drugs at a discounted price for use to treat common conditions. However, the ruling likely means that affected providers will be unable to enjoy 340B discounts for any orphan drug used for any purpose. The decision could prompt an exodus of providers from the program, invite additional legal challenges and ramp up pressure on Congress to intervene.
NCPA LTC Division Responds to Major CMS Proposed Rule Concerning Long Term Care Facilities: Earlier this summer, CMS released a proposed rule which contained major changes by updating conditions of participation for long term care facilities in the Medicare and Medicaid programs for the first time in nearly 25 years. The proposed changes are aimed to reduce hospital readmissions, reduce the risks associated with anti-psychotic medications and antibiotics, decrease the rate of infections and disease, and provide greater resident-centered care. There were several notable proposed changes to pharmacy services as well, including modifications to the drug regimen review process, care transitions, expanding the scope of psychotropic medications, and establishment of antibiotic stewardship programs. NCPA provided comments on behalf of our LTC members, and overall had concerns about added operational costs, as well as the overly broad definition of psychotropic medications which could include anti-hypertensives, anticonvulsants, and opioid analgesics. NCPA stated that while we appreciate the Agency’s efforts to improve quality of care, these proposed reforms need to be balanced with considerations of feasibility and implementation costs. Additionally, we believe a phased in approach of these provisions is appropriate to provide the industry with the time required to implement these changes.
PQA, Pharmacy Groups Hosting Quality Ratings Workshop for Community Pharmacists: The Pharmacy Quality Alliance (PQA) is hosting a one-day pharmacy quality ratings workshop for community pharmacists Nov. 9. It will focus on core competencies and techniques needed to perform well on quality measures currently used by health plans and PBMs to rate their pharmacy networks. The other hosts are the American Pharmacists Association, the National Association of Chain Drug Stores, NCPA, and the National Alliance of State Pharmacy Associations. The workshop will be held at the Westin Hotel in Old Town Alexandria, Va. Registration information and the full agenda may be found here.